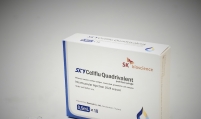
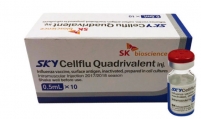
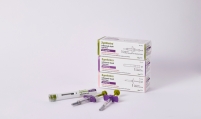
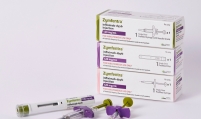
Bio
-
March 28, 2024
-
March 28, 2024
-
March 28, 2024
-
March 25, 2024
-
March 21, 2024
-
March 21, 2024
-
March 21, 2024
-
March 21, 2024
-
March 19, 2024
-
March 18, 2024
-
March 18, 2024
-
March 18, 2024
-
March 13, 2024
-
March 13, 2024
-
February 29, 2024